OUR SERVICES
We guide you through every phase of your product development |
|
The project manager is your dedicated interlocutor and follows personally all the steps from conception of the product until its introduction on the market.
He brings all necessary advice and provides a key link between all teams.
This provision in full service results in significant time saving for your project.
|
|
Our expertise to bring added value to your products |
|
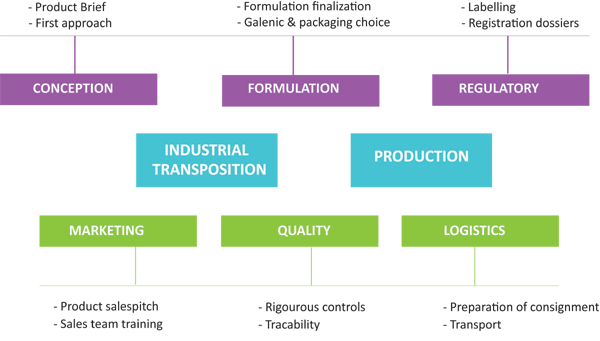
CONCEPTION |
|
REGULATORY |
|
PRODUCTION |
|
Scientists from R&D department, experts in nutrition and in cosmetics ensure a continuous technological watch on innovation, raw materials and on the latest ingredients.
They are thus able to propose innovative and competitive products both for developed formula or personalized products upon customer’s request.
|
|
From the conception phase of the project, the regulatory aspect is carefully studied in order to prevent possible difficulties during registration. In close consultation with customers, the regulatory team prepares all necessary documents including labelling. The whole dossier is then validated and certified by the pharmacist responsible for regulatory department.. |
|
To ensure competitiveness and quality, the product manager selects from its network the best equipped and most competent partners.This great industrial flexibility gives us the possibility to offer many galenic forms and those best suited. This specificity brings the company a great reactivity and a capacity of production almost unlimited. |
|
MARKETING |
|
QUALITY |
|
LOGISTICS |
|
|
At every step, our quality department is ensuring the strict compliance to the schedule of conditions defined and GMP procedures. For each batch produced, a batch dossier is made to garantee the perfect tracability of the product. Analyses of finished product are systematically carried out by independent laboratories. Samples of each batch are kept in our sample library. |
|
Every logistic aspect is taken into account thanks to the experience of Oligocaps Développement in International business. Export department provides all necessary documents for shipment. In order to simplify procedures, we can also deal with your forwarding agent or handle the whole transport to your warehouse. |